|
|
| Contributed Talk, Friday, 11:45 – 12:00 |
|
|
|
| Physical determinants of vascular
network remodeling during tumor growth
Michael Welter, Heiko Rieger
Saarland University, Theoretical
Physics, PF 151150, 66041 Saarbrücken, Germany |
|
|
| Contact:
| Website |
|
|
Tissues in living organisms need a persistent supply with oxygen and other
nutrients provided by the vascular blood flow through the vessel network
threading the tissue. Fast proliferating cells in a growing tumor have
an increased oxygen/nutrient demand, for which reason tumors usually cannot
grow beyond a size of 1-2mm3 without modifying the original vasculature.
This modification, comprising a substantial increase of microvascular density
(MVD) in the growth zone of the tumor, is denoted as angiogenesis, the
creation of new blood vessels from existing ones.
The emerging tumor vasculature is in many respects different from the
hierarchically organized arterio-venous blood vessel network in normal
tissues. The expected increase in MVD is usually observed in the periphery
of the tumor, whereas the morphology of the vasculature in the tumor center
is characterized by decreased MVD, dilated vessels, and regions of necrotic
tumor tissue. The resulting tumor-specific capillary network is very heterogeneous,
composed of dense and void regions, and has geometric properties different
from normal arterio-venous or normal capillary networks.
Besides pro- and anti-angiogenic molecular factors, mechanical, hydrodynamical
and collective processes must be involved in the process that transforms
or remodels the original arterio-venous blood vessel network into a tumor-specific
vasculature. In this paper we want, with the help of a theoretical model
[1-4] to address the physical determinants of the dynamical evolution,
final morphology and blood flow properties of an emerging tumor blood vessel
network. in which a growing tumor transforms a hierarchically organized
arterio-venous blood vessel network into a tumor specific vasculature is
analyzed. The determinants of this remodeling process involve the morphological
and hydrodynamic properties of the initial network, generation of new vessels
(sprouting angiogenesis), vessel dilation (circumferential growth), blood
flow correlated vessel regression, tumor cell proliferation and death,
and the interdependence of these processes via spatio-temporal changes
of blood flow parameters, oxygen / nutrient supply and growth factor concentration
fields. The emerging tumor vasculature is non-hierarchical and compartmentalized
into different zones. It displays a complex geometry with necrotic zones
and "hot spots" of increased vascular density and blood flow of varying
size. The origin of these hot spots is discussed. The blood vessel network
transports drug injections efficiently, but the computation of the interstitial
fluid flow shows that most of the drug is quickly washed out from the tumor
after extravasation.
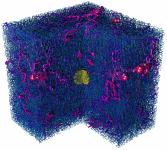
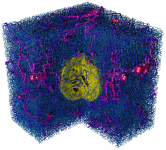
Fig. 1: Visualization of the vessel and tumor configuration generated
by a simulation of the model at different times (100h, 400h, 500h). A cut
through the cubic simulation volume is shown. The vessels are depicted
as cy-linders which are color coded by their blood pressure (blue = 0 kPa;
red = 12 kPa). Non-circulated vessels are shown in gray. The yellow spheroid
in the center shows the tumor with necrotic regions in the later stage.
| [1] |
|
D.-S. Lee, K. Bartha, H. Rieger,
Phys. Rev. Lett. 96: 058104 (2006). |
|
|
|
| [2] |
|
K. Bartha, H. Rieger, J. Theor.
Biol. 241: 903 (2006). |
|
|
|
| [3] |
|
M. Welter, K. Bartha, H. Rieger,
J. Theor. Biol. 259: 405 (2009). |
|
|
|
| [4] |
|
M. Welter, H. Rieger, Europ.
Phys. J. E 33: 149 (2010). |
|
|