|
|
| Contributed Talk, Thursday, 17:15 – 17:30 |
|
|
|
| Microfluidic Drops as Tunable
Bio-Environments
Christian Dammann, Bernd Nöding,
Sarah Köster
Institute for X-Ray Physics / Courant
Research Centre Nano-Spectroscopy and X-Ray Imaging, University of Göttingen,
Germany |
|
|
| Contact:
| Website |
|
|
Besides actin filaments and microtubules, intermediate filaments are a
major constituent of the eukaryotic cytoskeleton. Due to the importance
of the cytoskeleton for the mechanical properties of the cell, it is of
high interest to understand how this complex network is built up. In a
bottom up approach we focus on the assembly and network formation of vimentin
intermediate filaments in vitro. The assembly of vimentin depends
on the ionic strength of monovalent ions. By contrast, divalent ions act
as effective cross-linkers of vimentin networks [1,2]. We are able to directly
image the networks of the fluorescently tagged protein (Fig. 1b) and show
that divalent ions induce compaction of these networks. For that purpose,
we tailor a microfluidic device that allows for a systematic study of the
effect of magnesium on vimentin networks. In this device, a series of monodisperse
aqueous drops is created [3] and used as picoliter compartments for vimentin
(Fig. 1a). The drops are subsequently manipulated by changing flow rates
only. This manipulation process starts with the change of drop composition
from drop to drop, i.e. vimentin solution is encapsulated in drops with
tunable magnesium concentration. These drops are then stored in the device
for long-time observations by means of constrictions in the microfluidic
channel [4]. Our fluid manipulation method is designed to enable the reconstruction
of the content composition of each individual drop. Possible applications
of this tool are manifold, since our experimental method is not restricted
to encapsulate vimentin protein solution. For example, it can be used for
cell-based assays or for the study of other proteins. Due to the possibility
to define different concentrations of chemicals in a large number of drops,
hundreds of individual experiments are performed at the same time. This
feature predestines our method also for cell-based assays in drug discovery.
For instance, the response of cells to the concentration of a drug can
be studied at well-defined conditions while only a few microliters of the
cell solution and the drug are needed.
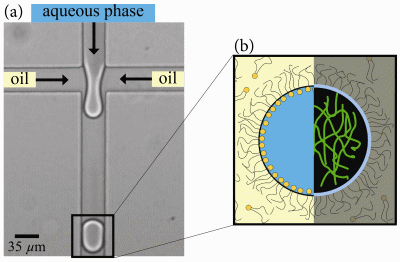
Fig. 1: We use the aqueous phase of a microemulsion as isolated
containers for the encapsulation of vimentin intermediate filaments. This
way, we study the effect of magnesium ions on vimentin networks by direct
imaging of the fluorescently tagged protein in the drops.
| [1] |
|
S. Köster, Y.-C. Lin, H. Herrmann,
D. A. Weitz: Nanomechanics of
Vimentin Intermediate Filament Networks, Soft Matter, 6: 1910-1914
(2010). |
|
|
|
| [2] |
|
Y.-C. Lin, N. Y. Yao, C. P. Broedersz,
H. Herrmann, F. C. MacKintosh, D. A. Weitz: Origins
of Elasticity in Intermediate Filament Networks, Phys. Rev. Lett.,
104: 058101 (2010). |
|
|
|
| [3] |
|
S. L. Anna, N. Bontoux, H. A. Stone: Formation
of Dispersions Using “Flow Focusing” in Microchannels, Appl. Phys.
Lett., 82: 364 (2003). |
|
|
|
| [4] |
|
C. H. J. Schmitz, A. C. Rowat, S. Köster,
D. A. Weitz: Dropspots: A Picoliter
Array in a Microfluidic Device, Lab Chip, 9: 44-49 (2009). |
|
|



